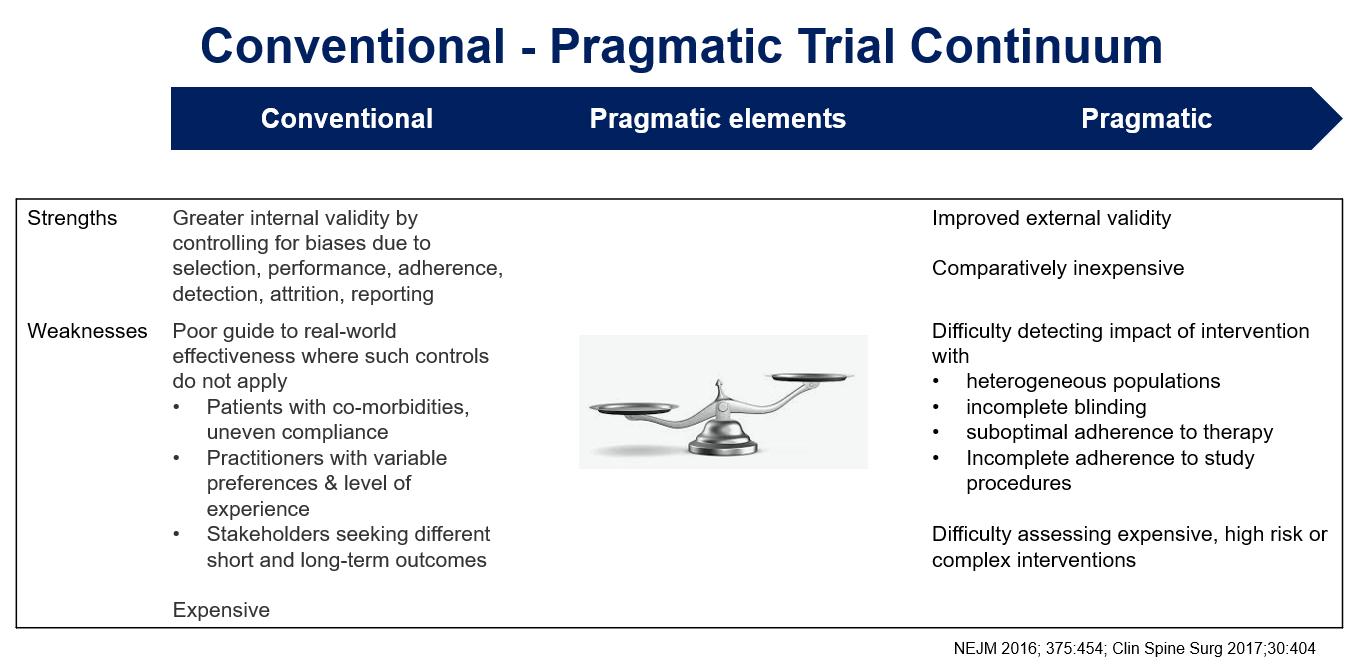
Conventional randomized trials confirm a physiological or clinical hypothesis.
- Such trials performed at sites with experienced investigators and highly selected participants may have limited clinical relevance and generalizability
Both types of studies must be of high quality
Conventional randomized trials confirm a physiological or clinical hypothesis.
Pragmatic trials should be strongly integrated with standard care, involve existing clinicians instead of dedicated research staff and use routinely collected clinical data
Evaluating strategies to accelerate the uptake of evidence-based research into clinical practice.
Sponsors from around the world have found the following benefits of using CPC's Endpoint Quality Intervention Program (EQuIP®) services:
CPC has developed the EQuIP® suite of services to decrease variability in functional endpoint testing, so you have definitive results at the end of the study. Taking into account your specific product, patient population, and intended purpose, this program starts with ensuring the best endpoints are chosen and the protocol is optimally designed to detect a signal. CPC then provides unique hands-on training at the study sites. All site staff that will be conducting endpoint testing for your study are required to perform mock tests with specially trained CPC team members to ensure consistent elements in data collection, methodologies, and practices. We supplement this training with on-line videos, training/reference tools and Core Lab worksheets.
During the study, sites submit information on how they conduct each test to our Core Lab. Our Core Lab reviews this information in real time, identifies issues, and provides feedback and recommendations for corrective action. This real time review allows us to recover data during the trial that would otherwise be lost. Additionally, CPC reviews monthly reports examining trends within and across countries, sites, and administrators in order to identify errors in test conduct and data that are not readily apparent when reviewing individual tests.
Choosing the right study design requires a full understanding of your development program objectives and specific study endpoints and their behavior in clinical trials. Ensuring that they are collected and adjudicated appropriately can make the difference between a successful trial and a failed trial. CPC brings worldwide Key Opinion Leaders (KOLs) and, decades of experience together to provide your trial with the scientific rigor and academic excellence you need for success. Services include:
Collecting endpoint data that involves active participation by the site staff and research subjects, requires rigorous quality control to determine the efficacy of your product. These same interactions inherently introduce variability. This standardization is an essential step in ensuring your endpoint is able to provide you with the answers you need.
CPC’s EQuIP addresses endpoint variability through:
While standard monitoring visits focus primarily on source document verification, protocol deviations, drug inventory, recruiting, and enrollment issues, SEEVs go beyond traditional monitoring and into the specifics of endpoint collection, ensuring that every detail is standardized and controlled at each clinical site. The outcome is results with integrity, data with low variability and decreased placebo response creating a decisive development path. SEEVs provide detailed review of the data collection methods, procedures, and equipment. Clinical site staff perform mock procedures with specially trained CPC team members to ensure consistent elements in collecting the critical data, methodologies, attitudes, and practices of the site staff.
CPC Clinical Research has developed innovative endpoint core labs in many different areas (e.g., Treadmill Testing, 6-Minute Walk, Pulmonary Function Testing (PFT), Wound Assessment, and more). Our endpoint core labs provide central and standardized solutions for reviewing data collection methods, evaluating data quality, and providing expert data interpretation.
The SQuAd Committee convenes frequently and allows quick and consistent problem solving to the unique endpoint issues that inevitably arise during trials. This committee includes Dr. William Hiatt, other clinical scientists, CPC project KOLs, Project Managers, and the CRAs who are doing the hands-on review of the endpoint data. This committee provides thoughtful solutions for consistent correction of site errors producing a higher degree of endpoint data quality.
As the experts in reducing variability in endpoint data collection, CPC can enhance your team’s knowledge of both the disease under study and how to ensure it is measured accurately. In conjunction with our other EQuIP services, our hands-on training programs provide your project team, when that team involves non-CPC staff, with tools and information that allow them to help identify problems early in the trial. We work collaboratively with your team to ensure you have the best quality data possible at the conclusion of your trial. In addition to our endpoint and disease specific training, CPC has developed a series of general clinical trials training courses for newly-hired clinical staff that need to be immediately productive.
To ensure an unbiased evaluation and verification of the analysis, it is optimal to have clinical trial results reviewed by an independent, academic research organization. CPC provides the independent, third-party review for your trial results. These independent analyses are often required by the major journals for publication.
Publication is important to your trial and important to CPC. One of our initiatives is to make information from EQuIP available to the public in the form of publications.